Myeloproliferative neoplasms (MPNs) are clonal malignant disorders of hematopoiesis arising in the hematopoietic stem cell (HSC) compartment that are characterized by excessive production of mature blood cells of the myeloid lineage. Transformation to secondary acute myeloid leukemia (sAML) represents a significant cause of death among MPN patients and this transformation occurs mainly from the clone carrying the disease phenotype driver mutation. Current treatment options for MPN patients are not curative and are limited to symptomatic treatment. Therefore, identification of novel therapeutic approaches with a clear disease-modifying effect for the treatment of MPNs and intercepting their progression to sAML is an unmet medical need. Mutations in JAK2, thrombopoietin receptor (MPL), and calreticulin (CALR) are phenotypic drivers in the pathogenesis of MPN. CALR mutations (CALRmut) are the second most frequent in MPN. CALRmut are insertions or deletions resulting in a frameshift in the last exon of the gene, causing a loss of the KDEL ER-retention motif and generation of a 36 amino acid positively charged C-terminal neoantigen. Due to loss of the KDEL motif, CALRmut are not confined to the ER and through interaction with MPL are trafficked to the cell surface where they induce persistent MPL activation and oncogenicity. Immunotherapies engaging T cells, such as bispecific cluster of differentiation 3 (CD3) redirection antibodies, show promising response rates in the clinic.
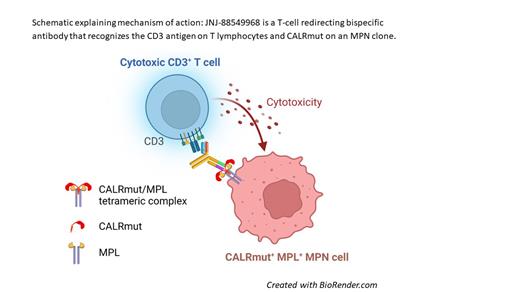
Here, we describe JNJ-88549968, a novel first in class T-cell redirecting bispecific antibody that selectively targets CALRmut with the potential to achieve cures by eliminating MPN clones. The mechanism of action of JNJ-88549968 is to act as a bridge between CALRmut MPN cancer cells and T cells, inducing T-cell activation with subsequent T-cell-mediated cytotoxicity to CALRmut cancer cells in vitro and in vivo. JNJ-88549968 recognizes CALRmut epitopes common to all known CALRmut types.
Cell surface localization of CALRmut was confirmed in CD34 + cells from CALRmut MPN patients. In-depth characterization of T cells from CALRmut MPN patients, using CyTOF and functional assays, confirmed their fitness and functionality. These data validate T-cell redirection as a rational therapeutic strategy for MPN patients carrying CALRmut.
JNJ-88549968 demonstrated selective binding to CALRmut cell lines and no measurable binding to CALR wild type cells. JNJ-88549968 led to CALRmut-selective T-cell activation and cytotoxicity to CALRmut-engineered cell lines in vitro. The activity of JNJ-88549968 was also explored in an autologous setting using CD34 + cells isolated from CALRmut MPN patients as target cells together with T cells isolated from the same patients. JNJ-88549968 elicited concentration-dependent cytotoxicity of patient-derived CALRmut CD34 + cells. JNJ-88549968-mediated cytotoxicity was observed against all tested CALRmut CD34 + cancer cells and was independent of the type of CALR mutation. Moreover, JNJ-88549968 mediated robust in vivo efficacy in two independent CALRmut-positive xenograft murine leukemia models. In an established disseminated model, treatment with JNJ-88549968 significantly increased lifespan (ILS) compared to vehicle-treated control mice.
Secreted CALRmut protein can be found in CALRmut MPN patient plasma. Evaluation of the impact of soluble CALRmut on JNJ-88549968 activity, including CALRmut-patient derived whole blood studies, indicated no effect on the activity of JNJ-88549968 in vitro.
Taken together, JNJ-88549968 is a novel first-in-class bispecific T-cell redirection antibody investigated for the treatment of CALRmut MPN. JNJ-88549968 is currently being advanced for clinical investigation in patients with MPN.
Disclosures
Kuchnio:Janssen: Current Employment, Current equity holder in publicly-traded company. Samakai:Janssen: Current Employment. Hug:MyeloPro Diagnostics and Research GmbH: Current Employment. Balmaña:MyeloPro Diagnostics and Research GmbH: Current Employment. Janssen:Janssen: Current Employment. Amorim:Janssen: Current Employment. Cornelissen:Janssen: Current Employment. Majoros:MyeloPro Diagnostics and Research GmbH: Current Employment. Broux:Janssen: Current Employment. Taneja:Janssen: Current Employment. Torti:Janssen: Current Employment. Agic:MyeloPro Diagnostics and Research GmbH: Current Employment. Moritsch:MyeloPro Diagnostics and Research GmbH: Current Employment. Benedetti:MyeloPro Diagnostics and Research GmbH: Current Employment. Rosebrock:MyeloPro Diagnostics and Research GmbH: Current Employment. Packman:Janssen: Current Employment, Current equity holder in publicly-traded company. Arts:Janssen: Current Employment, Current equity holder in publicly-traded company. Patel:Janssen: Current Employment. Lomas:Janssen: Current Employment, Current equity holder in publicly-traded company. Deyoung:Janssen: Current Employment, Current equity holder in publicly-traded company. Zagrijtschuk:MyeloPro Diagnostics and Research GmbH: Current Employment. Elsayed:Janssen: Current Employment, Current equity holder in publicly-traded company. Constantinescu:MyeloPro Diagnostics and Research GmbH: Other: Co-founder; Medscape, SAB GSK JAK2 inhibitor: Speakers Bureau. Kralovics:MyeloPro Diagnostics and Research GmbH: Other: co-founder. Philippar:Janssen: Current Employment, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal